Take part
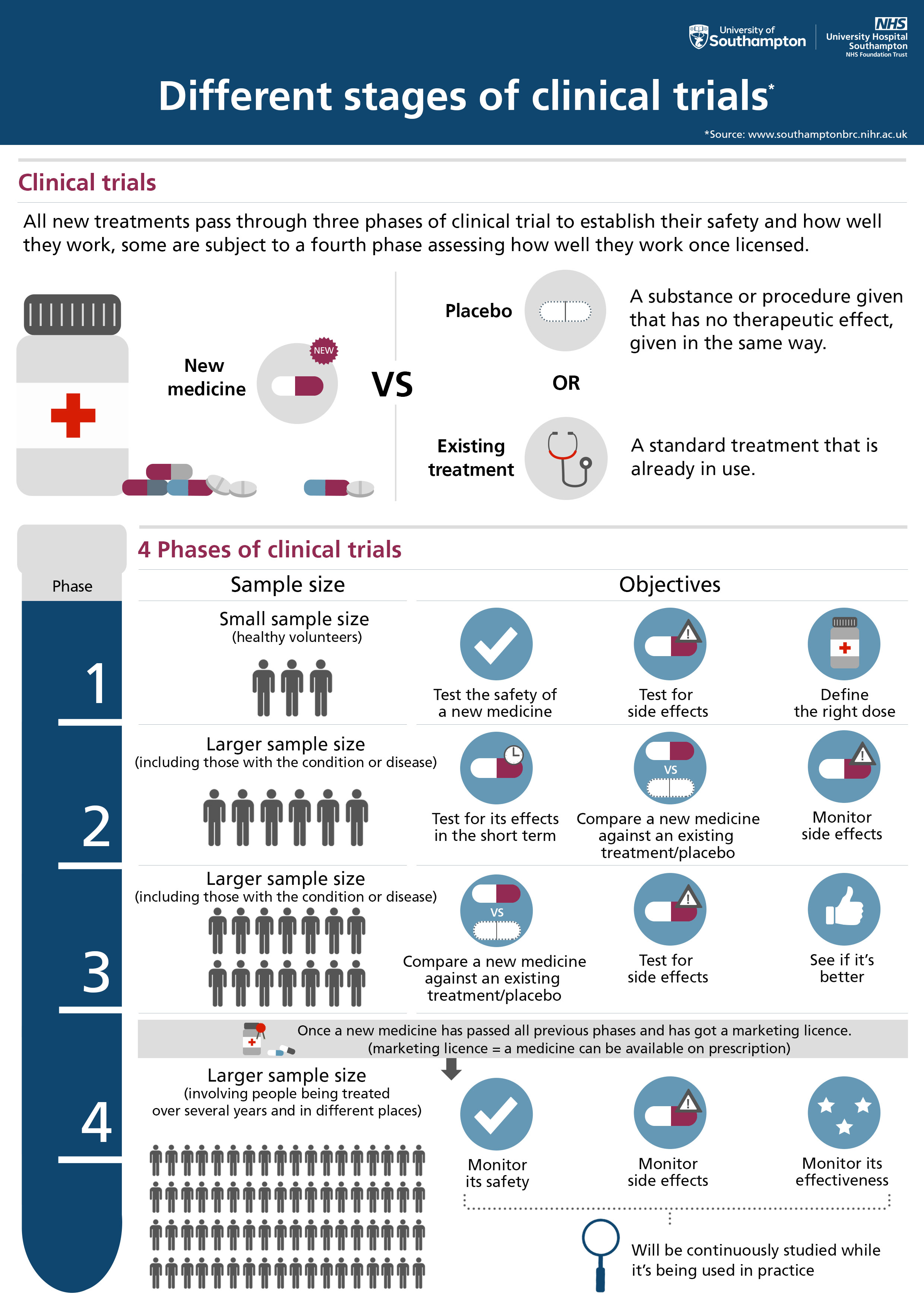
Clinical research studies and trials help us understand health better and develop new ways of treating and managing conditions, including asthma, diabetes, heart disease and many types of cancer. With hundreds of studies happening at any one time across a wide range of conditions, we are able to offer many people the opportunity to take part in research. Our research could not happen without patients and the public and, each year, we work with thousands of volunteers to conduct research and improve healthcare.
There are three ways you can help us:
Clinical research helps us better understand human health and wellbeing so we can:
- improve current treatments, medicines and care, and develop new and better ones
- diagnose diseases and conditions earlier or more accurately
- prevent people from developing diseases and conditions
Clinical research trials and studies are part of everyday work in the NHS and, you might not know it but, most care that patients receive in hospitals and GP practices is the result of research.
This includes experimental medicine trials, which look at the causes of disease, how treatments work and whether they are safe, and trials to test the effectiveness of new treatments to see if they are better than what is currently available.
Our research could not happen without patients and healthy volunteers and, each year, we work with thousands of volunteers to conduct research and uncover better ways to treat, prevent, diagnose and understand human disease.
People participate in trials and studies for a variety of reasons. Healthy volunteers might participate to help others and to contribute to moving science forward. Participants with an illness or disease also take part to help others, but also to possibly receive the newest treatment and to have the additional care and attention from clinical staff.
Trials and studies offer hope for many people and an opportunity to help researchers find better treatments for others. In many cases, the research will not help you personally, but it may provide vital information that will help people in the future.
All clinical research trials and studies are designed and carried out differently depending on what is being researched. This means what is involved for participants can also vary.
Some trials and studies involve regular tests, appointments and observations to test a current or new treatment, whilst others might involve completing questionnaires.
Before taking part in a trial or study make sure you understand its purpose, what is involved and any risks or benefits.
For more information, visit the NHS ‘clinical trials’ or the NIHR ‘be part of research’ pages.
As well as patients, many of our clinical research trials and studies aim to involve ‘healthy volunteers’ – people who are interested in contributing towards research but are generally healthy or do not have the condition or disease being investigated.
Healthy volunteers help researchers better understand a variety of health conditions as their information can be compared with people who have a specific disease or condition.
You can search for a trial or study currently recruiting healthy volunteers using our ‘trial finder’ below.
In Southampton, we have a database of healthy volunteers and send information about trials and studies they might be able to take part in. If you would like to join this database please contact the NIHR Southampton Clinical Research Facility (details below).
If you have any questions or would like more information, contact us by calling 023 8120 4989 or by emailing CRFstudyteam@uhs.nhs.uk.
You can ask your doctor or nurse if they know of any clinical trials or studies that you might be eligible to join. Or, patients and healthy volunteers can search for trials and studies using our ‘trial finder’ here.
All clinical research trials and studies are designed and carried out differently depending on what is being researched. This means potential risks to participants can also vary.
Treatments are thoroughly tested in laboratory trials before they are tested with groups of participants and, when trials and studies are designed, every effort is made to eliminate any risk to participants. Any known risks will be explained when you sign up.
The research team, doctors and nurses responsible for the research will monitor participants closely to detect any side effects. If there are changes, medical staff will act immediately.
In general, risks can include:
- not being able to choose which treatment you get
- the new treatment may not work for you
- more severe side effects than current treatments
Taking part in a clinical research trial or study is voluntary and up to each individual. You may choose not to take part or you may leave at any time.
Choosing not to take part or leaving the study will not result in any penalty and your decision will not affect the care you receive.
If you decide to change your mind after signing up, your information (including any samples already taken) may still be used as planned. If you do not wish for this to happen, speak with the trial or study team to see if it is possible for your information to be withdrawn.
Some clinical research trials and studies offer payment or expenses (including for travel), which can vary depending on what is involved. You will be told about any payments before you sign up.
It is important to find out about the risks and other commitments (such as the number of appointments and travelling distance) before you sign up, and to carefully weigh up whether it is worth it.
If you have found a clinical research trial or study that you would like to take part in, contact the team using the details provided.
Or, general enquiries can be made by contacting the NIHR Southampton Clinical Research Facility by calling 023 8120 4989 or emailing CRFstudyteam@uhs.nhs.uk.
In clinical research, Patient and Public Involvement (known as PPI) is defined as ‘clinical research carried out with or by members of the public, rather than about or for them’. This includes:
- meeting up to discuss ideas for clinical research trials and studies
- reviewing clinical research information sheets and posters
- feeding back ideas on how to promote clinical research trials and studies and their results
- helping us identify research priorities
- taking part in a clinical research trial or study steering group
See the ‘help shape our research’ section of our website for more information, and how you can get involved with Southampton’s PPI activities.
As an NHS organisation we use personally-identifiable information to conduct research to improve health, care and services. As a publicly-funded organisation, we have to ensure that it is in the public interest when we use personally-identifiable information from people who have agreed to take part in research. This means that when you agree to take part in a research study, we will use your data in the ways needed to conduct and analyse the research study. Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we have already obtained. To safeguard your rights, we will use the minimum personally-identifiable information possible.
When you agree to take part in a research study, the information about your health and care may be provided to researchers running other research studies in this organisation and in other organisations. These organisations may be universities, NHS organisations or companies involved in health and care research in this country or abroad. Your information will only be used by organisations and researchers to conduct research in accordance with the UK Policy Framework for Health and Social Care Research.
Your information could be used for research in any aspect of health or care, and could be combined with information about you from other sources held by researchers, the NHS or government.
Where this information could identify you, the information will be held securely with strict arrangements about who can access the information. The information will only be used for the purpose of health and care research, or to contact you about future opportunities to participate in research. It will not be used to make decisions about future services available to you, such as insurance.
Where there is a risk that you can be identified your data will only be used in research that has been independently reviewed by an ethics committee.
You can find out more about your information and health and care research here.
Health and care research should serve the public interest, which means that we have to demonstrate that our research serves the interests of society as a whole. We do this by following the UK Policy Framework for Health and Social Care Research.
If you wish to raise a complaint on how we have handled your personal data, you can contact our Data Protection Officer who will investigate the matter. If you are not satisfied with our response or believe we are processing your personal data in a way that is not lawful you can complain to the Information Commissioner’s Office (ICO).
If you'd like to find out we handle your data and how you can gain access to the information, visit our right of access pages. You can also contact the data protection office via dataprotection@uhs.nhs.uk or 020 8120 4743.
While most of our research is done on an opt in basis, participants also have the opportunity to opt out of our research studies should they wish to.
Please note that when research is conducted using de-personalised data, with no way for researchers to identify individual patients, explicit consent from patients for use of their data is not required. For more information, please refer to the ICO Guide to the General Data Protection Regulation (GDPR).
If you would prefer for your patient data not to be used for the research listed here, you can opt out using the contacts below.
For more information about opting out, please contact dataprotection@uhs.nhs.uk.
About opting out
As with all research collaboration agreements with non-NHS organisations, patients can opt out of any data-sharing system by emailing the trust’s data protection officer at dataprotection@uhs.nhs.uk. Patients will need to include their NHS or medical records number in any messages.
If you don't want your confidential patient information to be used for research and planning, you can opt out of this. If you do opt out, there are some specific situations where your data may still be used. Data that does not identify you may still also be used. In this case, all future research will take place excluding those who have opted out.
As with all studies on anonymised datasets, measures will be adopted to render it impossible to identify any individual patients. It is therefore not possible to opt out of research already underway for this reason.
ISARIC CCP
The CCP-UK (Clinical Characterisation Protocol – United Kingdom) is a study that collects information about infectious diseases and potential exposures of public health importance quickly and efficiently in response to potential public health crises. The study was activated in January 2020 in response to the emergence of COVID-19. Since being activated, we have recruited over 300,000 patients to the data collection aspect of our study. CCP-UK is the largest study of its kind answering questions about COVID-19 in the world. We have also been activated for UK cases of Ebola, Monkeypox, Lassa Fever, Middle East Respiratory Syndrome (MERS) and for Children with severe Hepatitis.
Please read this privacy notice for details on how patient data is used. For more information on ISARIC CCP, please visit the study website.
If you would like to opt out from your data being used for this study, please contact the study team to request this at ccp@liverpool.ac.uk or call 07506 653560. Include your name, date of birth, NHS number and postcode. You do not need to give a reason for why you want to opt out.
RIPCORD2
The RIPCORD 2 study is a collaboration between University Hospital Southampton (UHS) and Liverpool Heart and Chest Hospital (LHCH). The study is comparing two strategies for managing patients undergoing investigation for known or suspected problems in the heart arteries. Those who took part can read this privacy notice.
For more information about this, or should you wish to withdraw your data from the study, please contact Zoe Nicholas on 02381 208538 or email zoe.nicholas@uhs.nhs.uk.
Deciphering AMD by deep phenotyping and machine learning (PINNACLE)
Age-Related Macular Degeneration (AMD) is the commonest cause of blindness in the elderly. By 2020, 200 million people are expected to be affected with AMD, increasing to nearly 300 million by 2040. Unfortunately, doctors don’t know who will progress to the sight threatening stage of the disease. Some patients progress slowly or not at all and others quickly.
We can teach computers to analyse high resolution images of the inside of the eye. From UK Biobank and local eye clinics, we have access to hundreds of thousands of such images from people with AMD as well as those without. These images will allow us to train computers to identify what eye changes appear in patients with AMD. Alongside these images, we also have access to DNA results that we can match up to the image to see whether specific DNA changes affect the progression of AMD in individuals.
The records of patients over 50 years of age who have previously had retinal imaging performed in Southampton Eye Unit will be used for this study.
To opt out of this study, please contact PINNACLE@soton.ac.uk or call Janice Sutton on 023 8120 5049.
Investigation of Anaemia in Hospital (ISAIAH)
Currently there are no specific guidelines for doctors on how to diagnose and treat anaemia in hospital in the UK, with different approaches used depending on who treats the patient. The ISAIAH study aims to investigate current practices in a large teaching hospital, and develop new guidelines to ensure all anaemic patients receive the best possible care.
If you were diagnosed or treated for anaemia at any stage in 2016, anonymised data from your health records may be used in the analysis for in this study.
If you would prefer for your data not to be used in this study, please email Dr James Plumb at james.plumb@uhs.nhs.uk, call 023 8120 4989 or send a letter to: Clinical Research Facility, Southampton Centre for Biomedical Research, University Hospital Southampton NHS Foundation Trust, Mailpoint 218, Southampton General Hospital, Tremona Road, Southampton, SO16 6YD.
Improving major trauma triage
Clinicians in Southampton are evaluating a tool used by ambulance crews to decide on the most appropriate hospital destination for patients involved in traumatic incidents.
If you were taken by ambulance or air ambulance to Southampton General Hospital with traumatic injuries between 1 October 2016 and 30 September 2017, anonymised data from your health records may be used in the analysis in this study.
If you would prefer for your data not to be used in this study, please email Els Freshwater at els.freshwater@uhs.nhs.uk.
CHARIOT study follow-up
Researchers are looking at one-year outcomes and readmission rates of CHARIOT study participants, to see whether the tropinin test can predict future cardiac events. Those who took part can read this privacy notice.
This would allow researchers to use the test in the future to identify the groups who are at higher risk of having a cardiac event, and better target further care.
For more information about this, or should you wish to withdraw your data from the study, please contact the coronary research group on 023 8120 8538 or email jonathan.hinton@uhs.nhs.uk.
Troponin test review
Cardiac experts in Southampton are investigating whether a blood test that is used to diagnose heart muscle damage can help doctors predict the long-term health outcomes of intensive care patients and ensure they get the most appropriate treatment.
The test, which is known as a troponin test, measures levels of the protein ‘troponin’ in the blood. Normally troponin is present in blood in very small quantities but when there is damage to the heart muscle, such as during heart attack, it is released into the bloodstream causing levels to rise. A troponin test is used alongside an electrocardiogram (ECG) to help doctors determine if an individual has suffered a heart attack. However, raised troponin isn’t always the result of a heart attack and can be a sign of other forms of heart injury.
The study will analyse the routine blood tests and troponin levels of patients admitted to intensive care at Southampton General Hospital, with the aim of identifying what levels are considered ‘normal’ in this patient population.
Any patients admitted to intensive care at Southampton General Hospital during the study period who have a blood test will be included in this study. A troponin test will be added onto any blood remaining after the tests requested by the clinical team have been performed. This will be repeated through the intensive care stay. The research team will then observe the clinical progress of each patient to see if there is a relationship between the troponin level and patient outcomes. The research team will not perform any testing other than adding the troponin test onto left over blood that was taken by the clinical team. The clinical team can request the troponin test if clinically indicated but if the test was not clinically requested then the troponin result will be hidden from the clinical team. This study has provided useful clinical insights and as such the research team plan to assess the one year outcomes of this cohort using data obtained from NHS Digital. Those who took part in this study can read the privacy notice, which contains further information regarding this study.
For more information, or if you wish to withdraw your data from this study, please contact the coronary research group on 023 8120 8538, by emailing zoe.nicholas@uhs.nhs.uk or jonathan.hinton@uhs.nhs.uk, or by sending a letter to: Coronary research group, Cardiovascular and thoracic unit, Southampton General Hospital, Tremona Road, Southampton SO16 6YD.
Opt out of all studies
If you do not wish for your patient data to be used for any research studies or trials, please contact dataprotection@uhs.nhs.uk.
The study team were brilliant at reassuring me about any concerns I had before taking part. I'm proud of the fact I was able to be a part of this study.
Healthy volunteer